Norine
quinomycin B
- Norine ID: NOR01127
- DOI: 10.26097/nor01127
- Family: quinomycin
- Synonym(s):
quinomycin B;
- Activity: antimicrobial, antitumor
- Category:
peptide
- Formula: C53H68N12O12S2
- Monoisotopic mass: 1128.4521071000001 g/mol
- Comment: Quinomycins contain a thioacetal cross bridge. In the tiostin analogues, a disulfide bond replaces the thioacetal cross-link.
- Source: norine
- Contributor (creation): Norine Team
[CRIStAL (UMR CNRS 9189), ex-LIFL, France, Charles Viollette Institute, ProBioGEM team, Lille, France, University of Lille, France]
- Entry information:
- Type: double cyclic
- Number of monomers: 10
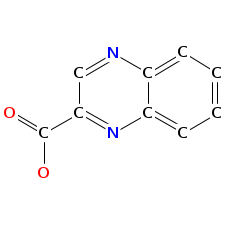
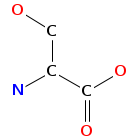
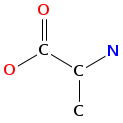
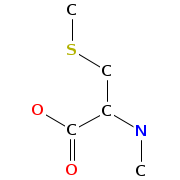
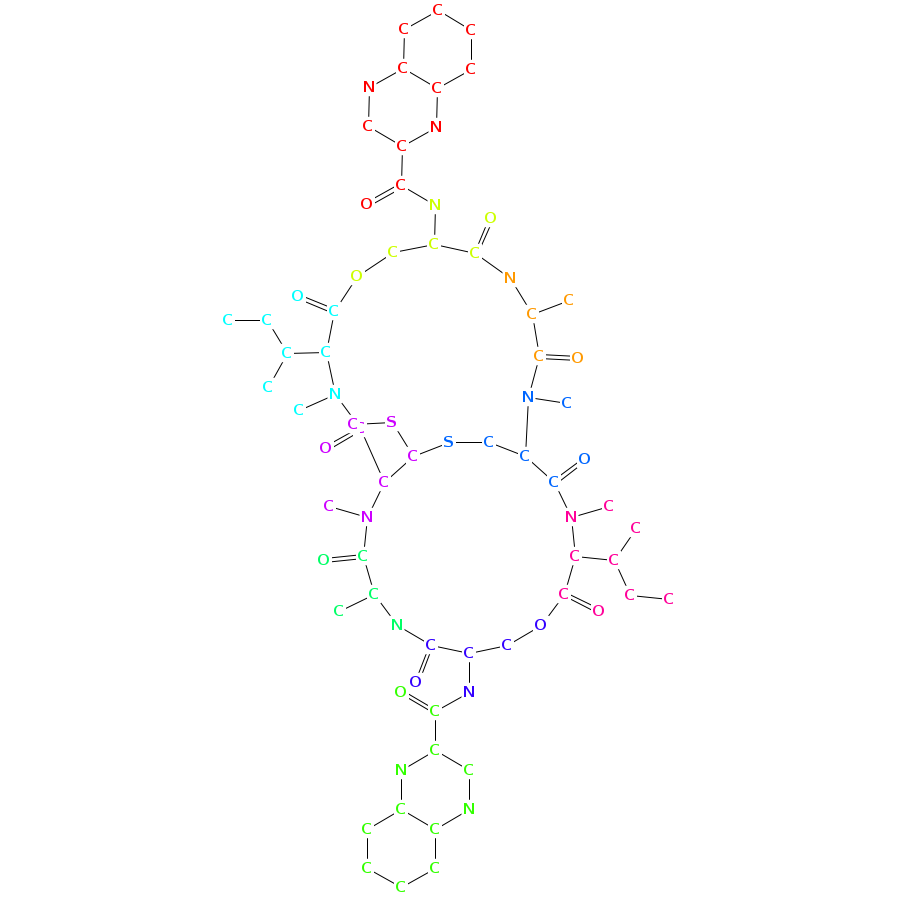
- Smiles: CCC(C)C1C(=O)OCC(C(=O)NC(C(=O)N(C2C(SCC(C(=O)N1C)N(C(=O)C(NC(=O)C(COC(=O)C(N(C2=O)C)C(C)CC)NC(=O)C3=NC4=CC=CC=C4N=C3)C)C)SC)C)C)NC(=O)C5=NC6=CC=CC=C6N=C5
- Graph inference:
- Monomeric composition :
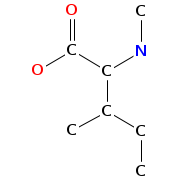
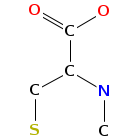
- Graph representation: COOH-Qui,D-Ser,Ala,diMe-Cys,NMe-aIle,D-Ser,COOH-Qui,Ala,NMe-Cys,NMe-aIle @1 @0,2,9 @1,3 @2,4,8 @3,5 @4,6,7 @5 @5,8 @3,7,9 @1,8
- Atomic structure:
 vizualisation:
vizualisation:
- Links between organisms producing the quinomycin B: Streptomyces sp.
- Influence of isoleucine upon quinomycin biosynthesis by Streptomyces sp. 732.
Journal of bacteriology , 1967, 93(4):1327-31
pubMed: 6032510
 Return to annotations search
Return to annotations search




 Return to annotations search
Return to annotations search