Norine
helvecardin A
- Norine ID: NOR00935
- DOI: 10.26097/nor00935
- Family: helvecardin
- Synonym(s):
helvecardin A;
- Activity: antimicrobial
- Category:
glycopeptide
- Formula: C90H103Cl2N9O36
- Monoisotopic mass: 1955.5882761529997 g/mol
- Comment: Helvecardins were strongly active against aerobic and anaerobic Gram-positive bacteria including methicillin-resistant Staphylococcus aureus, but they were inactive against Gram-negative bacteria and fungi. Two bonds of the central Hpg are due to oxidative ring closure reactions.
- Source: norine
- Contributor (creation): Norine Team
[CRIStAL (UMR CNRS 9189), ex-LIFL, France, Charles Viollette Institute, ProBioGEM team, Lille, France, University of Lille, France]
- Entry information:
- Type: other
- Number of monomers: 12
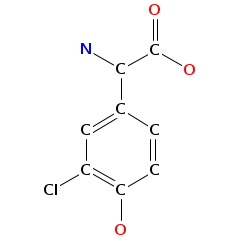
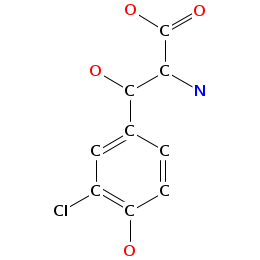
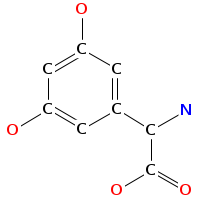
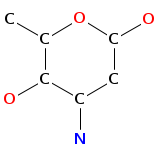
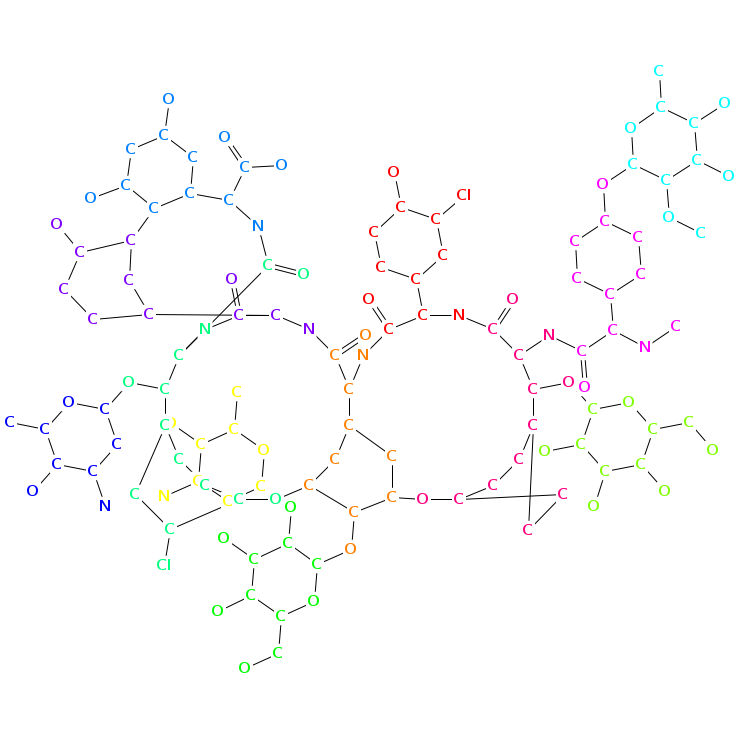
- Smiles: C[C@H]1[C@@H]([C@H](C[C@@H](O1)O[C@H]2[C@@H](O[C@@H]([C@H](C2O)O)CO)OC3=C4C=C5C=C3OC6=C(C=C(C=C6)[C@H]([C@H]7C(=O)N[C@@H](C8=CC(=CC(=C8C9=C(C=CC(=C9)[C@H](C(=O)N7)NC(=O)[C@@H]5NC(=O)C(NC(=O)[C@@H]([C@@H](C1=CC=C(O4)C=C1)OC1C(C(C(C(O1)CO)O)O)O)NC(=O)C(C1=CC=C(C=C1)OC1C(C(C(C(O1)C)O)O)OC)NC)C1=CC(=C(C=C1)O)Cl)O)O)O)C(=O)O)O[C@H]1C[C@@H]([C@H]([C@@H](O1)C)O)N)Cl)N)O
- Graph inference:
- Monomeric composition :
- Graph representation: NMe-Hpg,bOH-Tyr,D-Man,Hpg,Cl-Hpg,bOH-Cl-Tyr,Dhpg,Hpg,Ria,Ria,D-Glc,2OMe-Rha @1,11 @3,4,2,0 @1 @1,4,5,7,8 @1,3 @3,7,6,9 @7,5 @3,5,6 @3,10 @5 @8 @0
- Atomic structure:
 vizualisation:
vizualisation:
- Links between organisms producing the helvecardin A: Pseudonocardia compacta
 Return to annotations search
Return to annotations search


 Return to annotations search
Return to annotations search