Norine
lokisin
- Norine ID: NOR00342
- DOI: 10.26097/nor00342
- Family: amphisin
- Synonym(s):
anikasin;
- Activity: antimicrobial, surfactant
- Category:
lipopeptide
- Formula: C63H109N11O20
- Monoisotopic mass: 1339.785034983 g/mol
- Comment: Anikasin is a synonymous of lokisin. Only anikasin structure was experimentally elucidated.
- Source: norine
- Contributor (creation): Norine Team
[CRIStAL (UMR CNRS 9189), ex-LIFL, France, Charles Viollette Institute, ProBioGEM team, Lille, France, University of Lille, France]
- Entry information:
- Type: partial cyclic
- Number of monomers: 12
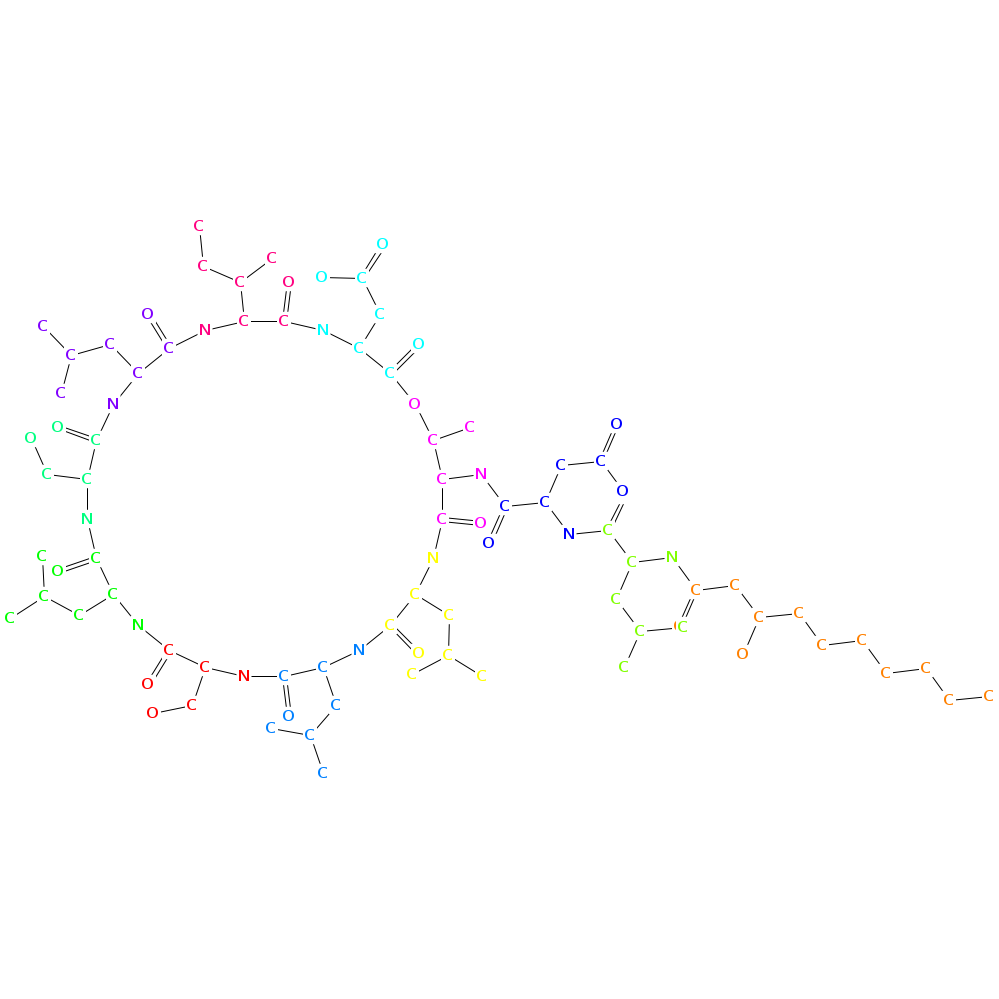
- Smiles: CCCCCCC[C@H](CC(=O)N[C@H](CC(C)C)C(=O)N[C@H](CC(=O)O)C(=O)N[C@@H]1[C@H](OC(=O)[C@@H](NC(=O)[C@H](NC(=O)C(NC(=O)[C@H](NC(=O)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC1=O)CC(C)C)CC(C)C)CO)CC(C)C)CO)CC(C)C)[C@H](C)CC)CC(=O)O)C)O
- Graph inference:
- Monomeric composition :
- Graph representation: C10:0-OH(3),D-Leu,D-Asp,D-aThr,D-Leu,D-Leu,D-Ser,Leu,D-Ser,Leu,Ile,Asp @1 @0,2 @1,3 @2,4,11 @3,5 @4,6 @5,7 @6,8 @7,9 @8,10 @9,11 @3,10
- Atomic structure:
 vizualisation:
vizualisation:
- Links between organisms producing the lokisin: Pseudomonas sp. DSS41, Pseudomonas fluorescens HKI0770
- Cyclic lipoundecapeptide lokisin from Pseudomonas sp. strain DSS41
Nielsen TH,
Christophersen C,
Sorensen J,
Sorensen D,
Tetrahedron letters , 2002, 43 4421-4423.
- Structure, Biosynthesis, and Biological Activity of the Cyclic Lipopeptide Anikasin
Götze S,
Klapper M,
Stallforth P,
Herbst-Irmer R,
Görls H,
Schneider KRA,
Barnett R,
Burks T,
Neu U,
ACS Chemical Biology , 2017, 12:2498-2502
DOI: 10.1021/acschembio.7b00589
pubMed: 28846366
 Return to annotations search
Return to annotations search
.png)





 Return to annotations search
Return to annotations search