Norine
hemiasterlin A
- Norine ID: NOR00457
- DOI: 10.26097/nor00457
- Family: hemiasterlin
- Synonym(s):
hemiasterlin A;
- Activity: antitumor, toxin
- Category:
peptide
- Formula: C29H44N4O4
- Monoisotopic mass: 512.336255916 g/mol
- Source: norine
- Contributor (creation): Norine Team
[CRIStAL (UMR CNRS 9189), ex-LIFL, France, Charles Viollette Institute, ProBioGEM team, Lille, France, University of Lille, France]
- Entry information:
- Type: linear
- Number of monomers: 3
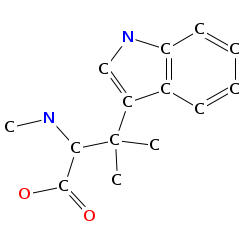
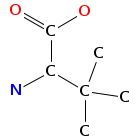
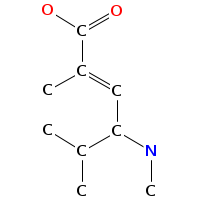
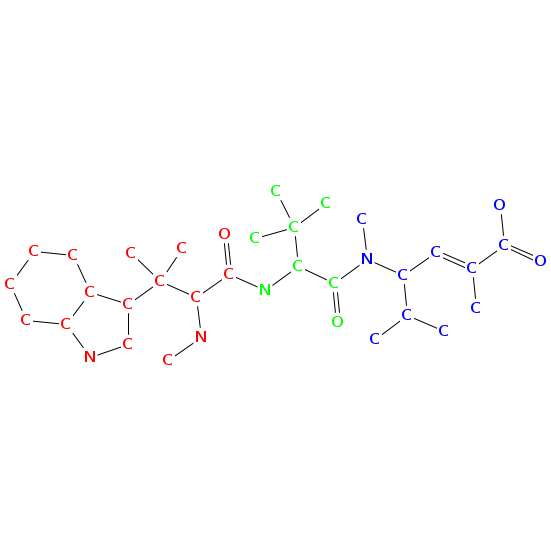
- Smiles: CNC(C(=O)NC(C(=O)N(C)C(C=C(C)C(=O)O)C(C)C)C(C)(C)C)C(C)(C)c2c[nH]c1ccccc12
- Graph inference:
- Monomeric composition :
- Graph representation: bbMe-NMe-Trp,t-Leu,NMe-hv-Val @1 @0,2 @1
- Atomic structure:
 vizualisation:
vizualisation:
- Links between organisms producing the hemiasterlin A: Auletta sp., Siphonochalina spp., Cymbastela
- Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation
Andersen RJ,
Anderson HJ,
Coleman JE,
Roberge M,
Cancer chemotherapy and pharmacology , 1997, 39(3):223-6.
DOI: 10.1007/s002800050564
pubMed: 8996524
- Cytotoxic and tubulin-interactive hemiasterlins from Auletta sp. and Siphonochalina spp. sponges
Hamel E,
Boyd MR,
Gamble WR,
Durso NA,
Fuller RW,
Westergaard CK,
Johnson TR,
Sackett DL,
Cardellina JH,
Bioorganic and medicinal chemistry , 1999, Aug,7(8):1611-5.
DOI: 10.1016/s0968-0896(99)00089-9
pubMed: 10482453
 Return to annotations search
Return to annotations search
 Return to annotations search
Return to annotations search